The activated reaction of dichlorocarbene with triplet molecular oxygen†
Abstract
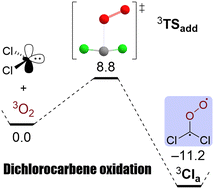
The well-known dichlorocarbene (CCl2, 1) is deemed to undergo an extremely facile addition reaction with triplet molecular oxygen (3O2) under formation of the corresponding singlet Criegee intermediate, phosgene O-oxide. This is unexpected, because the carbene possesses a singlet ground state with a large singlet–triplet gap and, typically, only triplet carbenes react swiftly with triplet dioxygen. Hence, we deployed a careful theoretical study of this reaction and computed the oxygen addition barrier at levels of electron correlation as high as CCSD(T) and BD(TQ) and basis sets as large as cc-pV5Z. Our results firmly establish the existence of a reaction barrier, and we estimate its height to amount to 8.8 kcal mol−1. Furthermore, the initially formed triplet dioxygen adduct is prone to facile O–O bond breaking rendering phosgene and triplet oxygen atoms likely products of the overall reaction. As a general conclusion, we find that carbenes are ambiphiles in oxygen additions and more electrophilic as well as that more nucleophilic carbenes show greater reactivity.
- This article is part of the themed collection: Benchmark Experiments for Numerical Quantum Chemistry


 Please wait while we load your content...
Please wait while we load your content...