Reduced nucleophilicity: an intrinsic property of the Lewis base atom interacting with H in hydrogen-bonds with Lewis acids HX (X = F, Cl, Br, I, CN, CCH, CP)†
Abstract
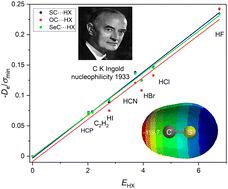
Equilibrium hydrogen-bond dissociation energies De for the process B⋯HX = B + HX are calculated at the CCSD(T)(F12c)/cc-pVDZ-F12 level for ∼190 complexes B⋯HX. As established earlier, De values for such complexes can be described by the equation De = cNBEA, in which NB and EHX are the nucleophilicity and electrophilicity of the Lewis base B and the Lewis acid HX, respectively, and the constant c = 1 kJ mol−1. Graphs of De as the ordinate and EHX as the abscissa are presented for 26 series of hydrogen-bonded complexes B⋯HX. The Lewis base is fixed and HX is HF, HCl, HBr, HI, HCN, HCCH and HCP in each series. Each plot yields a good straight line, the slope of which is the nucleophilicity NB of B. The Lewis bases are chosen for their simplicity and all have at least one non-bonding electron pair carried by the atom directly involved in the B⋯HX hydrogen bond. The direction of the minimum value σmin of the molecular electrostatic surface potential on the 0.001 e bohr−3 iso-surface in the chosen bases B usually coincides with the axis of a non-bonding electron pair. The gradient of a graph of De/σmin plotted against EHX defines a reduced nucleophilicity ИB = NB/σmin in the sense that ИB appears to be a property only of the atom of B that is directly involved in the B⋯HX hydrogen bond, independent of the remainder of B. For example, the values of the reduced nucleophilicity for the series of isocyanide complexes CH3NC⋯HX, HNC⋯HX and FNC⋯HX are 0.0343(16), 0.0337(18) and 0.0332(18), respectively, while those for the corresponding series of cyanide complexes are 0.0337(23), 0.0329(24) and 0.0333(23).
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...