The impact of size and shape in the performance of hydrotropes: a case-study of alkanediols†
Abstract
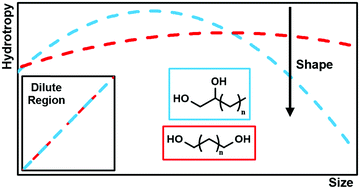
Inspired by the recently proposed cooperative mechanism of hydrotropy, where water molecules mediate the aggregation of hydrotrope around the solute, this work studies the impact of apolar volume and polar group position on the performance of hydrotropes. To do so, the ability of two different families of alkanediols (1,2-alkanediols and 1,n-alkanediols) to increase the aqueous solubility of syringic acid is initially investigated. Interestingly, it is observed that in the dilute region (low hydrotrope concentration), the relative position of the hydroxyl groups of the alkanediols does not impact their performance. Instead, their ability to increase the solubility of syringic acid correlates remarkably well with the size of their alkyl chains. However, this is not the case for larger hydrotrope concentrations, where 1,2-alkanediols are found to perform, in general, better than 1,n-alkanediols. These seemingly contradictory findings are reconciled using theoretical and experimental techniques, namely the cooperative model of hydrotropy and chemical environment probes (Kamlet–Taft and pyrene polarity scales). It is found that the number of hydrotropes aggregated around a solute molecule does not increase linearly with the apolar volume of the former, reaching a maximum instead. This maximum is discussed in terms of competing solute–hydrotrope and hydrotrope–hydrotrope interactions. The results suggest that hydrotrope self-aggregation is more prevalent in 1,n-alkanediols, which negatively impacts their performance as hydrotropes. The results reported in this work support the cooperative model of hydrotropy and, from an application perspective, show that hydrotropes should be designed taking into consideration not only their apolar volume but also their ability to stabilize their self-aggregation in water, which negatively impacts their performance as solubility enhancers.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...