Theoretical study of NiI–NiIII cycle mediated by heterogeneous zinc in C–N cross-coupling reaction†
Abstract
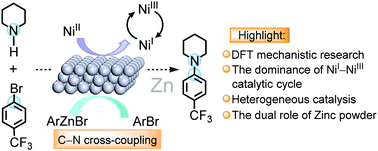
Photoredox/transition-metal dual catalysis could efficiently construct C–N bonds by a cross-coupling reaction. The limitations of low recovery, low utilization rate and high cost have hindered the application and development of low-cost and efficient transition metal catalytic cycles. The integration of heterogeneous metal and transition metal catalysis is an appealing alternative to realize the oxidation state modulation of active species. With the support of density functional theory (DFT) calculation, we have explored the mechanistic details of Ni-catalyzed C–N cross-coupling of aryl bromide and cyclic amine assisted by zinc powder. Zinc successfully regulates the oxidation state of NiII → NiI, thus achieving the NiI–NiIII–NiI catalytic cycle in the absence of light. In comparison, when the Ni(0) complex is employed as the initial catalyst, organic zinc reagents can still be involved in the transmetalation process to accelerate the cross-coupling reaction. We hope that such computational studies can provide theoretical reference for the design and development of low-cost and efficient catalytic systems for C–N cross-couplings.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...