Effect of transition metal cations on the local structure and lithium transport in disordered rock-salt oxides†
Abstract
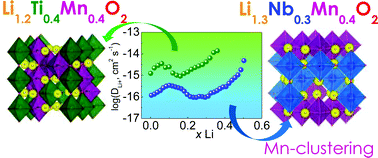
Lithium-excess oxides Li1.2Ti0.4Mn0.4O2 and Li1.3Nb0.3Mn0.4O2 with a disordered rock-salt structure and Mn3+/Mn4+ as a redox couple were compared to analyze the effect of different d0 metal ions on the local structure and Li+ ion migration. These cathode materials were obtained by mechanochemically assisted solid-state synthesis. Using XRD, 7Li NMR and EPR spectroscopy and transmission electron microscopy it was shown that the Mn ions are prone to form clusters, while d0 metal ions are evenly distributed in the crystal lattice. The presence of Nb5+ ions contributes to the formation of noticeably larger Mn clusters and larger gaps in the Li+ migration maps as compared to Ti4+. These results were confirmed by the geometrical–topological method, BVSE simulation and DFT calculations, and are in good agreement with the Li diffusion coefficient determined by GITT, which is 1.5 orders of magnitude higher in Li1.2Ti0.4Mn0.4O2 than that in Li1.3Nb0.3Mn0.4O2.


 Please wait while we load your content...
Please wait while we load your content...