Electronic structure, stability, and aromaticity of M2B6 (M = Mg, Ca, Sr, and Ba): an interplay between spin pairing and electron delocalization†
Abstract
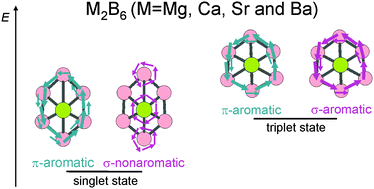
It has been shown in previous studies that the Be2B6 complex exhibits a triplet ground state with double aromaticity. In this work, the stability, electronic structure, and aromaticity of the homologous series M2B6 (M = Mg, Ca, Sr and Ba) were examined and compared to those of Be2B6. At the CCSD(T)/def2-TZVP//B3LYP/def2-TZVP level of theory, the target molecules were found to be more stable in the singlet than in the triplet spin state. Magnetically induced current densities and multicentre delocalization index (MCI) were employed to assess the aromatic character of the studied complexes. Both employed methods agree that M2B6 (M = Mg, Ca, Sr and Ba) are π aromatic and σ nonaromatic in the singlet ground state, and double aromatic in the triplet state. It was demonstrated that the electron counting rules of aromaticity cannot be used to correctly predict the aromaticity and relative stability of the examined molecules in different spin states.


 Please wait while we load your content...
Please wait while we load your content...