The effect of separation of blocks on the crystallization kinetics and phase composition of poly(butylene adipate) in multi-block thermoplastic polyurethanes†
Abstract
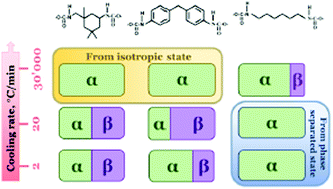
The influence of the hard segment nature on the crystallization kinetics of multi-block thermoplastic polyurethanes containing poly(butylene adipate) (PBA) as a soft segment was investigated. Using a combination of FTIR spectroscopy, time-domain 1H nuclear magnetic resonance (TD-NMR), differential scanning calorimetry (DSC), fast-scanning calorimetry (FSC) and wide-angle X-ray diffraction (WAXS), it was shown that aliphatic, cycloaliphatic and aromatic diisocyanates affect the phase separation efficiency of soft and hard segments. The best phase separation efficiency was observed for a sample containing aliphatic diisocyanate due to the development of a hydrogen bond network. The thermal history, phase separation and the degree of ordering of the polyurethane determine the polymorphic behavior of melt-crystallized PBA. The formation of a partially-ordered mesophase of linear aliphatic polyurethane leads to an increase in the crystallization rate of PBA at room temperature and the formation of thermodynamically stable α-crystals. The presence of bulk cycloaliphatic and aromatic diol-urethane fragments prevents the phase separation of PBA, which crystallizes after slow cooling in a mixture of α- and β-crystalline forms. The new nanocalorimetry technique allows the identification of a direct correlation between the phase separation and crystallization kinetics of the melt-crystallized PBA in a wide range of cooling rates – from 2 to 30 000 K s−1. Particularly, ultra-fast cooling suppresses the nucleation of the β-phase of PBA resulting in slow crystallization of only α-modification at room temperature. The role of the polyurethane mesophase in the crystallization of the soft segment was discussed for the first time.


 Please wait while we load your content...
Please wait while we load your content...