Alkyne insertion into Cu–Al bonds and selective functionalization to form copper acyl compounds†
Abstract
We report on the insertion of alkynes into heterometallic M–M′ bonds, producing (aluminylalkenyl)copper compounds which possess differential reactivity at the two derived M–C functions. Uniquely, this system is capable of controlling access to isolable syn or anti dimetallated alkenes, by employing either kinetic or thermodynamic control. Subsequent derivatization with CO is both stereoselective (to syn systems) and regioselective (to Cu–C bonds), leading to the formation of the first structurally characterized examples of copper acyl compounds - aided by the cooperative reactivity of the proximal aluminium centre.
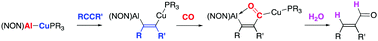


 Please wait while we load your content...
Please wait while we load your content...