Selective synthesis and (chir)optical properties of binaphthyl-based chiral carbon macrocycles†
Abstract
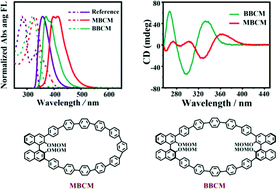
Herein, we report the selective synthesis, characterization, and photophysical properties of two novel chiral carbon macrocycles. Non-planar (S)-2,2′-bis(methoxymethoxy)-1,1′-binaphthalene was introduced into the scaffold of oligo-paraphenylenes to achieve the chirality in these macrocycles. Their photophysical properties were investigated by steady-state and time-resolved spectroscopies, as well as circular dichroism and circularly polarized luminescence spectroscopies. We demonstrate that the emission maxima of the chiral macrocycles are redshifted compared to chiral binaphthyl units and that macrocycles show chiroptical properties (|glum| > 1.0 × 10−3).


 Please wait while we load your content...
Please wait while we load your content...