Dithienoazaborine derivatives with selective π-conjugated extension via late-stage functionalization†
Abstract
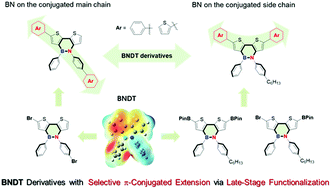
The selective late-stage functionalization of BN-containing polycyclic aromatic hydrocarbons (BN-PAHs) is very challenging. Herein, dithienoazaborines (BNDT, 3a and 3b) were synthesized as model compounds, and two kinds of selective functionalized BNDT precursors, 4a and 4b were synthesized due to the strong polar effect of the BN bond, leading to a series of BNDT derivatives for the following coupling reactions. Based on this, two kinds of BNDT-based small molecules and oligomers with BN bonds on the conjugated main chain and side chain were synthesized accordingly. The BNDT derivatives (5b/6b/7b) with a BN bond on the conjugated side chain showed lower LUMO energy levels and narrower band gaps than those with a BN bond on the conjugated main chain (5a/6a/7a). Different modifications and polymerization have effects on the photophysical properties and all derivatives are highly selective to fluoride ions. This work realized a new strategy for selective late-stage functionalization of BN-PAHs with selective π-conjugated extension, laying a solid foundation for the subsequent BN chemistry and application of functionalized BN-PAHs.


 Please wait while we load your content...
Please wait while we load your content...