Homoleptic cyclometalated dibenzothiophene–NHC–iridium(iii) complexes for efficient blue phosphorescent organic light-emitting diodes†
Abstract
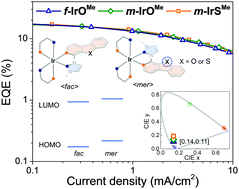
The NHC–Ir complexes f-IrSiPr, m-IrSiPr, and m-IrSMe, in which a dibenzothiophene (DBT) moiety is used to increase the emission efficiency for deep-blue phosphorescence, were synthesized and compared with the dibenzofuran (DBF)-based Ir complexes f-IrOMe and m-IrOMe. The differences in the ligand structure (DBF/DBT) or configuration (fac/mer) of these complexes led to different electrochemical and photophysical properties. The DBF moiety has a stronger electronegativity than DBT, resulting in a larger T1–S0 energy gap and a shorter emission wavelength than those of the DBT complexes. On the other hand, the meridional isomer has a mutually trans-phenyl ligand configuration that leads to lengthening of the transoid Ir–C bond and destabilizes the HOMO level, resulting in greater ease of oxidation, and the emission is red-shifted relative to the facial forms. Even with the differences in the origin of phosphorescence, replacement of the oxygen atom in the DBF unit with sulfur does not greatly alter the emission efficiency in either solution or film while achieving the same high-end deep-blue phosphorescence with unprecedented CIE coordinates of [0.14, 0.19] for m-IrSMe (EQEmax; 17.1%) and [0.14, 0.14] for m-IrOMe (EQEmax; 18.2%).


 Please wait while we load your content...
Please wait while we load your content...