A solid electrolyte interphase to protect the sulfurized polyacrylonitrile (SPAN) composite for Li–S batteries: computational approach addressing the electrolyte/SPAN interfacial reactivity†
Abstract
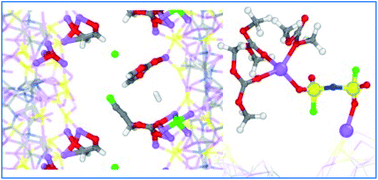
This study addresses the reactivity of multiple solvents and lithium bis(fluorosulfonyl)imide (LiFSI) at the interface with sulfurized polyacrylonitrile (SPAN) in multiple stages of lithiation via ab initio molecular dynamics simulations. Both ether 1,3-dioxolane (DOL) and dimethyl carbonate (DMC) proved stable on the lithiated SPAN surface regardless of the lithium content, meaning that neither of these species likely contributes to growing a solid electrolyte interphase (SEI) coating to protect the SPAN composite. Conversely, cyclic carbonates, ethylene carbonate (EC) and fluoroethylene carbonate (FEC) are shown to be very active. The EC reduction occurs only on a highly lithiated surface with a 3.0 Li/S molar ratio, the equivalent of the SPAN composite in an over-discharge regime with voltages close to 0.0 V vs. Li/Li+. The FEC reduction starts with a 2.0 Li/S molar ratio and above, suggesting that FEC could act as a useful additive in electrolyte formulations with EC. Both EC and FEC follow multiple reduction mechanisms to produce complex reduction products and LiF in the FEC case. We provide a mechanistic description for each detected decomposition path. The LiFSI salt also proves reactive against the lithiated SPAN surface. The FSI− defluorination is the dominant reduction path. However, the SO2NSO2F− and SO2NSO22− species proved stable against S–N cleavage. This behavior makes the LiFSI salt a potential candidate for SPAN-based Li–S batteries because it produces LiF without releasing SO2.


 Please wait while we load your content...
Please wait while we load your content...