Experimental and theoretical investigations of methyl orange adsorption using boron nitride nanosheets
Abstract
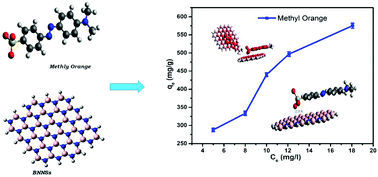
Fractions of dyes, that are used in large quantities for various applications, are lost during the dying process and contaminate water. In order to avoid their harmful effect on human health, boron nitride nanosheets (BNNSs) have been synthesized in this work and their adsorption behavior for the removal of anionic methyl orange (MO) dye from aqueous solution has been reported. The effect of pH, contact time, and initial dye concentration has been investigated on MO to find the optimum pH, equilibrium and adsorption capacity of the synthesized BNNSs. The adsorption kinetics and isotherm models showed that pseudo-second-order (PSO) kinetic and Freundlich isotherm models were being followed during the adsorption, respectively. The experimental maximum adsorption capacity of the synthesized adsorbent was found to be 575.0 mg g−1, which is due to the strong electrostatic attraction between the negatively charged MO and positively charged BNNSs. Furthermore, density functional theory (DFT) calculations have also been performed to investigate the nature and feasibility of the adsorption process, the interactions of MO dye molecules with the adsorbent, and the adsorption capacity of BNNSs. The theoretical and experimental studies suggest that the adsorption process is physical in nature. It was found that negative charge transfer occurred from MO to BNNSs with high chemical potential suggesting high chemical activity and a decrease in band gap after the adsorption process. These theoretical and experimental findings demonstrate the potential of BNNSs as adsorbents for commercial applications.


 Please wait while we load your content...
Please wait while we load your content...