Dipole–dipole interactions control the interfacial rheological response of cyclodextrin/surfactant solutions
Abstract
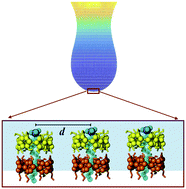
A recent surface rheological study has shown that aqueous solutions of α-cyclodextrin (αCD) with anionic surfactants (S) display a remarkable viscoelasticity at the liquid/air interface, which has not been observed in similar systems. The dilatational modulus is various orders of magnitude larger than those for the binary mixtures αCD + water and S + water. The rheological response has been qualitatively related to the bulk distribution of species, the 2 : 1 inclusion complexes (αCD2 : S) playing a fundamental role. In this work, we have developed a model that considers dipole–dipole interactions between 2 : 1 inclusion complexes ordered on the liquid/air interface. When the model is applied to the specific experimental conditions, the dependencies on concentration and temperature of the dilatational modulus and the surface tension were found to be in excellent agreement with the data, indicating clearly that dipole–dipole interactions determine and control the rheological behavior of the interface.


 Please wait while we load your content...
Please wait while we load your content...