Bispidine as a β-strand nucleator: from a β-arch to self-assembled cages and vesicles†
Abstract
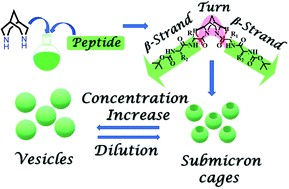
The development of synthetic scaffolds that nucleate well-folded secondary structures is highly challenging. Herein, we designed and synthesized a series of core-modified peptides (F1, F2, F3, and F4) that fold into β-strand structures. These bispidine-scaffolded peptides were studied by CD, IR, NMR, single crystal XRD, and Molecular Dynamics (MD) simulations to investigate their conformational preferences. Solid-state and solution studies revealed that bispidine is a versatile scaffold that could be placed either at the terminal or at the middle of the peptide strand for nucleating the β-strand structure. Scaffolds that nucleate an isolated β-strand conformation are rare. Bispidine placed at the C-terminus of the peptide chain could nucleate a β-strand conformation, while bispidine placed at the middle resulted in a β-arch conformation. This nucleation activity stems from the ability to restrict the psi torsion angle (ψ) through intramolecular C5 hydrogen bonding between the equatorial hydrogen(s) of bispidine and the carbonyl oxygen(s) of the amino acid close to the scaffold. Furthermore, the bispidine peptidomimetic with a super secondary structure, namely β-arch, assembled into single-hole submicron cages and spherical vesicles as evident from microscopic studies. The design logic defined here will be a significant strategy for the development of β-strand mimetics and super secondary structures.


 Please wait while we load your content...
Please wait while we load your content...