Three-component 1,2-carboamination of vinyl boronic esters via amidyl radical induced 1,2-migration†
Abstract
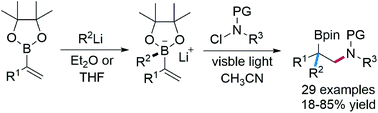
Three-component 1,2-carboamination of vinyl boronic esters with alkyl/aryl lithium reagents and N-chloro-carbamates/carboxamides is presented. Vinylboron ate complexes generated in situ from the boronic ester and an organo lithium reagent are shown to react with readily available N-chloro-carbamates/carboxamides to give valuable 1,2-aminoboronic esters. These cascades proceed in the absence of any catalyst upon simple visible light irradiation. Amidyl radicals add to the vinylboron ate complexes followed by oxidation and 1,2-alkyl/aryl migration from boron to carbon to give the corresponding carboamination products. These practical cascades show high functional group tolerance and accordingly exhibit broad substrate scope. Gram-scale reaction and diverse follow-up transformations convincingly demonstrate the synthetic potential of this method.


 Please wait while we load your content...
Please wait while we load your content...