A chromophore-supported structural and functional model of dinuclear copper enzymes, for facilitating mechanism of action studies†
Abstract
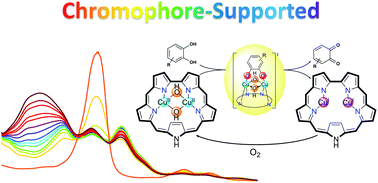
Type III dicopper centres are the heart of the reactive sites of enzymes that catalyze the oxidation of catechols. Numerous synthetic model complexes have been prepared to uncover the fundamental chemistry involved in these processes, but progress is still lagging much behind that for heme enzymes. One reason is that the latter gain very much from the informative spectroscopic features of their porphyrin-based metal-chelating ligand. We now introduce sapphyrin-chelated dicopper complexes and show that they may be isolated in different oxidation states and coordination geometries, with distinctive colors and electronic spectra due to the heme-like ligands. The dicopper(I) complex 1-Cu2 was characterized by 1H and 19F NMR spectroscopy of the metal-chelating sapphyrin, the oxygenated dicopper(II) complex 1-Cu2O2 by EPR, and crystallographic data was obtained for the tetracopper(II)-bis-sapphyrin complex [1-Cu2O2]2. This uncovered a non-heme [Cu4(OH)4]4− cluster, held together with the aid of two sapphyrin ligands, with structural features reminiscent of those of catechol oxidase. Biomimetic activity was demonstrated by the 1-Cu2O2 catalyzed aerobic oxidation of catechol to quinone; the sapphyrin ligand aided very much in gaining information about reactive intermediates and the rate-limiting step of the reaction.


 Please wait while we load your content...
Please wait while we load your content...