Chemoenzymatic glycan-selective remodeling of a therapeutic lysosomal enzyme with high-affinity M6P-glycan ligands. Enzyme substrate specificity is the name of the game†
Abstract
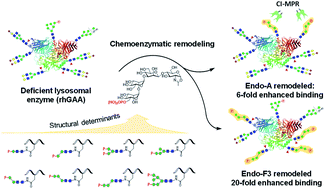
Functionalization of therapeutic lysosomal enzymes with mannose-6-phosphate (M6P) glycan ligands represents a major strategy for enhancing the cation-independent M6P receptor (CI-MPR)-mediated cellular uptake, thus improving the overall therapeutic efficacy of the enzymes. However, the minimal high-affinity M6P-containing N-glycan ligands remain to be identified and their efficient and site-selective conjugation to therapeutic lysosomal enzymes is a challenging task. We report here the chemical synthesis of truncated M6P-glycan oxazolines and their use for enzymatic glycan remodeling of recombinant human acid α-glucosidase (rhGAA), an enzyme used for treatment of Pompe disease which is a disorder caused by a deficiency of the glycogen-degrading lysosomal enzyme. Structure–activity relationship studies identified M6P tetrasaccharide oxazoline as the minimal substrate for enzymatic transglycosylation yielding high-affinity M6P glycan ligands for the CI-MPR. Taking advantage of the substrate specificity of endoglycosidases Endo-A and Endo-F3, we found that Endo-A and Endo-F3 could efficiently deglycosylate the respective high-mannose and complex type N-glycans in rhGAA and site-selectively transfer the synthetic M6P N-glycan to the deglycosylated rhGAA without product hydrolysis. This discovery enabled a highly efficient one-pot deglycosylation/transglycosylation strategy for site-selective M6P-glycan remodeling of rhGAA to obtain a more homogeneous product. The Endo-A and Endo-F3 remodeled rhGAAs maintained full enzyme activity and demonstrated 6- and 20-fold enhanced binding affinities for CI-MPR receptor, respectively. Using an in vitro cell model system for Pompe disease, we demonstrated that the M6P-glycan remodeled rhGAA greatly outperformed the commercial rhGAA (Lumizyme) and resulted in the reversal of cellular pathology. This study provides a general and efficient method for site-selective M6P-glycan remodeling of recombinant lysosomal enzymes to achieve enhanced M6P receptor binding and cellular uptake, which could lead to improved overall therapeutic efficacy of enzyme replacement therapy.


 Please wait while we load your content...
Please wait while we load your content...