Mechanically compliant single crystals of a stable organic radical†
Abstract
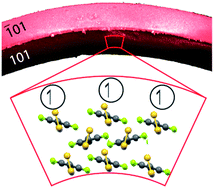
Mechanically compliant organic crystals are the foundation of the development of future flexible, light-weight single-crystal electronics, and this requires reversibly deformable crystalline organic materials with permanent magnetism. Here, we report and characterize the first instance of a plastically bendable single crystal of a permanent organic radical, 4-(4′-cyano-2′,3′,4′,5′-tetrafluorophenyl)-1,2,3,5-dithiadiazolyl. The weak interactions between the radicals render single crystals of the β phase of this material exceedingly soft, and the S–N interactions facilitate plastic bending. EPR imaging of a bent single crystal reveals the effect of deformation on the three-dimensional spin density of the crystal. The unusual mechanical compliance of this material opens prospects for exploration into flexible crystals of other stable organic radicals towards the development of flexible light-weight organic magnetoresistance devices based on weak, non-hydrogen-bonded interactions in molecular crystals.


 Please wait while we load your content...
Please wait while we load your content...