Synthesis of aza-quaternary centers via Pictet–Spengler reactions of ketonitrones†
Abstract
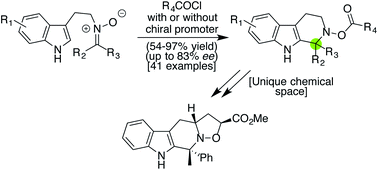
Despite the array of advances that have been made in Pictet–Spengler chemistry, particularly as it relates to the synthesis of β-carboline derivatives of both natural and designed origin, the ability to use such reactions to generate aza-quaternary centers remains limited. Herein, we report a simple procedure that enables the synthesis of a variety of such products by harnessing the distinct reactivity profiles of ketonitrones as activated by commercially available acyl chlorides. Notably, the reaction process is mild, fast, and high-yielding (54–97%) for a diverse collection of substrates, including some typically challenging ones, such as indole cores with electron-deficient substituents. In addition, by deploying an acyl bromide in combination with a thiourea promoter, a catalytic, asymmetric version has been established, leading to good levels of enantioselectivity (up to 83% ee) for several ketonitrones. Finally, the resultant N–O bonds within the products can also be functionalized in several unique ways, affording valuable complementarity to existing Pictet–Spengler variants based on the use of imines.


 Please wait while we load your content...
Please wait while we load your content...