Mechanism of inhibition of SARS-CoV-2 Mpro by N3 peptidyl Michael acceptor explained by QM/MM simulations and design of new derivatives with tunable chemical reactivity†
Abstract
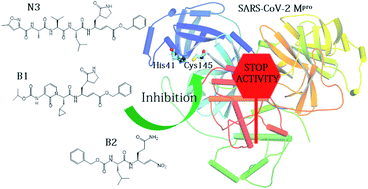
The SARS-CoV-2 main protease (Mpro) is essential for replication of the virus responsible for the COVID-19 pandemic, and one of the main targets for drug design. Here, we simulate the inhibition process of SARS-CoV-2 Mpro with a known Michael acceptor (peptidyl) inhibitor, N3. The free energy landscape for the mechanism of the formation of the covalent enzyme-inhibitor product is computed with QM/MM molecular dynamics methods. The simulations show a two-step mechanism, and give structures and calculated barriers in good agreement with experiment. Using these results and information from our previous investigation on the proteolysis reaction of SARS-CoV-2 Mpro, we design two new, synthetically accessible N3-analogues as potential inhibitors, in which the recognition and warhead motifs are modified. QM/MM modelling of the mechanism of inhibition of Mpro by these novel compounds indicates that both may be promising candidates as drug leads against COVID-19, one as an irreversible inhibitor and one as a potential reversible inhibitor.
- This article is part of the themed collections: Coronavirus articles - free to access collection and Most popular 2021 physical and theoretical chemistry articles, 2021


 Please wait while we load your content...
Please wait while we load your content...