Construction of chiral α-tert-amine scaffolds via amine-catalyzed asymmetric Mannich reactions of alkyl-substituted ketimines†
Abstract
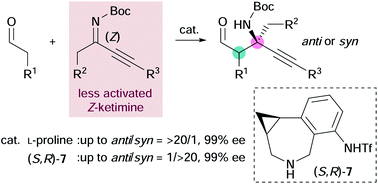
Stereoselective Mannich reactions of aldehydes with ketimines provide chiral β-amino aldehydes that bear an α-tert-amine moiety. However, the structural variation of the ketimines is limited due to the formation of inseparable E/Z isomers, low reactivity, and other synthetic difficulties. In this study, a highly diastereodivergent synthesis of hitherto difficult-to-access β-amino aldehydes that bear a chiral α-tert-amine moiety was achieved using the amine-catalyzed Mannich reactions of aldehydes with less-activated Z-ketimines that bear both alkyl and alkynyl groups.
- This article is part of the themed collection: 2021 Nobel Prize in Chemistry – Asymmetric Organocatalysis


 Please wait while we load your content...
Please wait while we load your content...