Synthesis of an elusive, stable 2-azaallyl radical guided by electrochemical and reactivity studies of 2-azaallyl anions†
Abstract
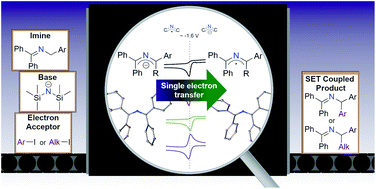
The super electron donor (SED) ability of 2-azaallyl anions has recently been discovered and applied to diverse reactivity, including transition metal-free cross-coupling and dehydrogenative cross-coupling processes. Surprisingly, the redox properties of 2-azaallyl anions and radicals have been rarely studied. Understanding the chemistry of elusive species is the key to further development. Electrochemical analysis of phenyl substituted 2-azaallyl anions revealed an oxidation wave at E1/2 or Epa = −1.6 V versus Fc/Fc+, which is ∼800 mV less than the reduction potential predicted (Epa = −2.4 V vs. Fc/Fc+) based on reactivity studies. Investigation of the kinetics of electron transfer revealed reorganization energies an order of magnitude lower than commonly employed SEDs. The electrochemical study enabled the synthetic design of the first stable, acyclic 2-azaallyl radical. These results indicate that the reorganization energy should be an important design consideration for the development of more potent organic reductants.


 Please wait while we load your content...
Please wait while we load your content...