Using internal electrostatic fields to manipulate the valence manifolds of copper complexes†
Abstract
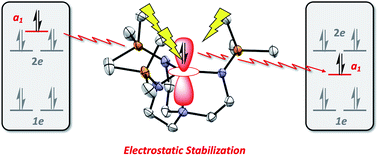
A series of tetradentate tris(phosphinimine) ligands (R3P3tren) was developed and bound to CuI to form the trigonal pyramidal, C3v-symmetric cuprous complexes [R3P3tren-Cu][BArF4] (1PR3) (PR3 = PMe3, PMe2Ph, PMePh2, PPh3, PMe2(NEt2), BArF4 = B(C6F5)4). Electrochemical studies on the CuI complexes were undertaken, and the permethylated analog, 1PMe3, was found to display an unprecedentedly cathodic CuI/CuII redox potential (−780 mV vs. Fc/Fc+ in isobutyronitrile). Elucidation of the electronic structures of 1PR3via density functional theory (DFT) studies revealed atypical valence manifold configurations, resulting from strongly σ-donating phosphinimine moieties in the xy-plane that destabilize 2e (dxy/dx2−y2) orbital sets and uniquely stabilized a1 (dz2) orbitals. Support is provided that the a1 stabilizations result from intramolecular electrostatic fields (ESFs) generated from cationic character on the phosphinimine moieties in R3P3tren. This view is corroborated via 1-dimensional electrostatic potential maps along the z-axes of 1PR3 and their isostructural analogues. Experimental validation of this computational model is provided upon oxidation of 1PMe3 to the cupric complex [Me3P3tren-Cu][OTf]2 (2PMe3), which displays a characteristic Jahn–Teller distortion in the form of a see-saw, pseudo-Cs-symmetric geometry. A systematic anodic shift in the potential of the CuI/CuII redox couple as the steric bulk in the secondary coordination sphere increases is explained through the complexes' diminishing ability to access the ideal Cs-symmetric geometry upon oxidation. The observations and calculations discussed in this work support the presence of internal electrostatic fields within the copper complexes, which subsequently influence the complexes' properties via a method orthogonal to classic ligand field tuning.


 Please wait while we load your content...
Please wait while we load your content...