Rapid formation of 2-lithio-1-(triphenylmethyl)imidazole and substitution reactions in flow†
Abstract
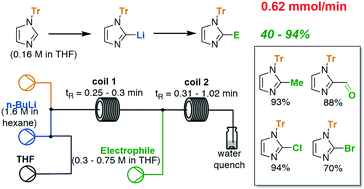
The functionalisation of imidazoles is a necessary step in the formation of many active pharmaceutical intermediates. Herein, we report a flow chemistry approach for the rapid and efficient formation of 2-lithio-1-(triphenylmethyl)imidazole at ambient temperature and its reaction with a range of electrophiles, achieving modest to high yields (40–94%) in short reaction times (<1 min). The method is amenable to the scale-up of this highly reactive lithio-imidazole intermediate.


 Please wait while we load your content...
Please wait while we load your content...