New total synthesis and structure confirmation of putative (+)-hyacinthacine C3 and (+)-5-epi-hyacinthacine C3†
Abstract
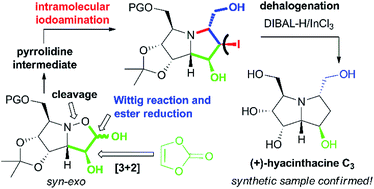
A unique synthesis of polyhydroxylated pyrrolizidine alkaloids, namely (+)-hyacinthacine C3 and (+)-5-epi-hyacinthacine C3 is presented. The strategy relies on a 1,3-dipolar cycloaddition of an L-mannose derived nitrone, which owing to its great syn-stereoselectivity builds up the majority of the required stereocenters. The following key steps include Wittig olefination and iodine-mediated aminocyclisation, that provide two epimeric pyrrolizidines with the appropriate configuration. As a result, structure and steric arrangement of the first synthetically prepared (+)-hyacinthacine C3 are proved to be correct, clearly confirming the inconsistency with the stereochemistry assigned to the natural sample. With respect to the previously proven glycosidase inhibitory activities, the antiproliferative effect of (+)-hyacinthacine C3 and (+)-5-epi-hyacinthacine C3 was evaluated using several cell line models.


 Please wait while we load your content...
Please wait while we load your content...