Recent trends in dehydroxylative trifluoro-methylation, -methoxylation, -methylthiolation, and -methylselenylation of alcohols
Abstract
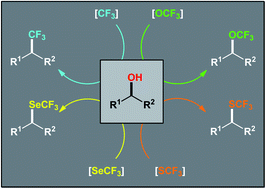
Owing to the prevalence of hydroxyl groups on molecules, much attention has been paid to the synthesis of functionalized organic compounds by dehydroxylative functionalization of parent alcohols. In this context, dehydroxylative trifluoromethylation, trifluoromethoxylation, trifluoromethylthiolation, and trifluoromethylselenylation of readily available alcohols have recently emerged as intriguing protocols for the single-step construction of diverse structures bearing C–CF3, C–OCF3, C–SCF3, and C–SeCF3 bonds, respectively. This Mini-Review aims to summarize the major progress and advances in this appealing research area with special emphasis on the mechanistic features of the reaction pathways.


 Please wait while we load your content...
Please wait while we load your content...