Synthesis of tertiary alkyl fluorides and chlorides by site-selective nucleophilic ring-opening reaction of α-aryl azetidinium salts†
Abstract
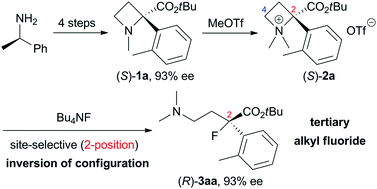
Site-selective nucleophilic ring-opening reactions of 2-arylazetidine-2-carboxylic acid ester-derived tetraalkyl ammonium salts 2 with tetrabutylammonium halides (Bu4NX) to give tertiary alkyl halides are successfully demonstrated. For example, a nucleophilic ring-opening reaction of 2-(o-tolyl) derivative 2a with 1.2 equivalents of tetrabutylammonium fluoride (Bu4NF) in THF at 60 °C preferentially proceeded at a more substituted carbon atom (2-position) compared to a less-substituted carbon atom (4-position) and afforded tert-butyl 4-(dimethylamino)-2-fluoro-2-(o-tolyl)butanoate 3aa in 71% yield as the corresponding tertiary alkyl fluoride. This result was applied to synthesize optically active organofluorine compounds starting from commercially available (R)-1-phenylethylamine.


 Please wait while we load your content...
Please wait while we load your content...