Deoxygenative nucleophilic difluoromethylselenylation of carboxylic acids and alcohols with BT-SeCF2H†
Abstract
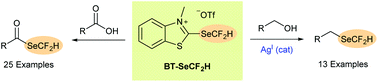
The benzothiazolium salt BT-SeCF2H is introduced as an efficient nucleophilic reagent for transferring difluoromethylselenyl groups onto organic molecules. SeCF2H-Containing selenoesters could be prepared upon deoxygenative substitution of readily available carboxylic acids, while silver catalysis allowed for efficient formation of (difluoromethyl)selenoethers, including the established electrophilic reagent BnSeCF2H, directly from simple alcohols. To the best of our knowledge, these deoxygenative reactions represent the first reported nucleophilic difluoromethylselenylation processes and thus open up new approaches to prepare valuable fluorinated compounds.
- This article is part of the themed collection: Recent Open Access Articles in Frontiers Journals


 Please wait while we load your content...
Please wait while we load your content...