Photocatalytic decarboxylative alkylation of silyl enol ether and enamide with N-(acyloxy)phthalimide using ammonium iodide†
Abstract
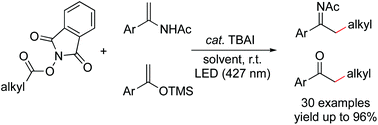
Enamides and silyl enol ethers were alkylated to deliver products of N-acyl imines and ketones in the presence of a catalytic amount of N-tetrabutylammonium iodide (TBAI) under irradiation with purple light-emitting diodes (427 nm) at room temperature. A broad scope of tertiary, secondary, and primary alkylation products was achieved with good yields and tolerance of a variety of functional groups. The reactions proceed through the photoactivation of a transiently assembled electron donor–acceptor complex formed between iodide and N-(acyloxy)phthalimide to generate alkyl radicals. The simplicity and low-cost nature of these methods without using metal catalysts demonstrate the practicality of the use of alkyl carboxylates as alkyl sources in organic synthesis.


 Please wait while we load your content...
Please wait while we load your content...