Alkali-amide-catalyzed divergent sp2 and sp3 C–H bonds alkylation of alkylthiophenes with alkenes†
Abstract
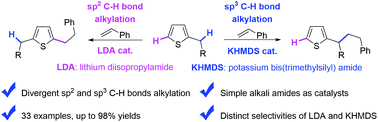
The divergent transformations of different C–H bonds in a given substrate represent the state of the art in C–H functionalization reactions, wherein there is a need for precise control of the selectivity of the catalyst. Simply by using alkali amide catalysts, we herein selectively achieved the addition of divergent sp2 and sp3 C–H bonds of alkylthiophenes to alkenes. Lithium diisopropylamide (LDA) underwent the deprotonation of the C5 sp2 C–H bond and addition to alkenes to afford C5 alkylation products. Potassium bis(trimethylsilyl) amide (KHMDS) failed to undergo the deprotonation but established a deprotonative equilibrium between the C5 sp2 C–H bond and the benzylic sp3 C–H bonds. Further alkylation with alkenes, however, selectively took place at the benzylic sp3 C–H bond. This work presents the divergent alkylation products of alkylthiophenes, and demonstrates the interesting and distinct behaviors of potassium and lithium amides.


 Please wait while we load your content...
Please wait while we load your content...