Revised structural assignment of azalomycins based on genomic and chemical analysis†
Abstract
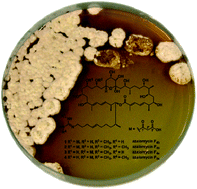
Actinobacteria have long served as sources of novel natural products with antibiotic properties, but the isolation of novel derivatives and the determination of their absolute structures have remained a scientific challenge. The objective of this study was to clarify the absolute structures of the azalomycin core structure as previous structural assignments remained controversial. Based on the isolation of four azalomycin derivatives (F4a, F4b, F5a and F5b) from the termite-associated Streptomyces sp. M56, we applied 1D and 2D nuclear magnetic resonance (NMR), J-based configuration analyses (JBCA), electronic circular dichroism (ECD), high-resolution (HR)-electrospray ionization (ESI) mass spectrometry to clarify the stereochemical assignment. These studies were supported by in silico studies of all ketoreductase and enoylreductase domains encoded within the PKS. The combined analysis allowed us to propose a revised stereochemical assignment of the four azalomycin derivatives, and bioactivity studies confirmed and expanded the antifungal and antiproliferative properties of azalomycins.


 Please wait while we load your content...
Please wait while we load your content...