Effect of noncovalent interactions in ion pairs on hypervalent iodines: inversion of regioselectivity in sulfonyloxylactonization†
Abstract
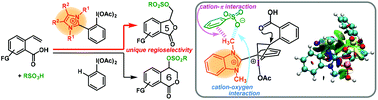
We synthesized novel hypervalent iodines possessing cationic heterocyclic moieties nearby the iodine(III) center. The novel hypervalent iodines exhibited a totally different regioselectivity from common PhI(OAc)2 during the sulfonyloxylactonization of 2-vinylbenzoic acids. The noncovalent interactions between the sulfonyloxy groups and the cationic heterocyclic moieties resulted in a significant change in the regioselectivity, which was revealed by the observation of intermediates and density functional theory studies including noncovalent interaction analysis.


 Please wait while we load your content...
Please wait while we load your content...