N-Alkyl nitrones as substrates for access to N-aryl isoxazolidines via a catalyst-free one-pot three-component reaction with nitroso compounds and olefins†
Abstract
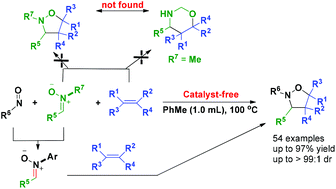
N-Alkyl nitrones are used as the starting materials to construct various N-aryl isoxazolidines, instead of N-alkyl isoxazolidines or N–H 1,3-oxazinanes via a catalyst-free one-pot three-component reaction with nitroso compounds and olefins. The mechanism investigations indicate that the in situ formation of N-aryl nitrones from the reaction of N-alkyl nitrones and nitrosoarenes is the key step. The protocol features broad substrate scope, good to excellent chemo-, regio- and diastereo-selectivities, and moderate to excellent yields for most substrates.


 Please wait while we load your content...
Please wait while we load your content...