Dynamic covalent bond constrained ureas for multimode fluorescence switching, thermally induced emission, and chemical signaling cascades†
Abstract
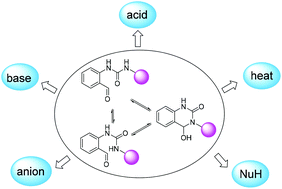
Urea groups play an important role in organic, supramolecular, and materials chemistry owing to unique structural and molecular recognition features. Herein a strategy of reversible covalent bond constrained ureas was developed toward multi-addressable switchable luminescent molecules and materials. The combination of ortho urea and aldehyde led to equilibrating cyclic hemiaminals, and the fluorescence response can be tuned through multimodes, including acid, base, anion, temperature, and nucleophiles. Moreover, mechanism studies revealed the critical role of an emissive aromatic acyliminium ion intermediate in acidic media, enabling triple-state ON–OFF–ON switching by changing pH. Through controlling the dynamic system thermally activated fluorescence and tunable dynamic covalent reactions with nucleophiles were further achieved. The unique reactivity of the acyliminium ion intermediate also allowed the creation of proton relay mediated signaling cascades toward the regulation of fluorescence outputs. To demonstrate the utility multi-stimuli luminescence switching and the detection of various vapors were realized in the solid state. The current platform provides a new pathway for manipulating urea bonds and should be appealing to future research of assembly, sensing, and labelling.


 Please wait while we load your content...
Please wait while we load your content...