Construction of the tetracyclic core of the Lycopodium alkaloid annotinolide C†
Abstract
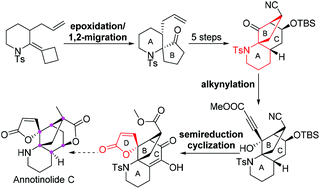
A concise method of synthesizing the tetracyclic core of annotinolide C has been developed. The key steps include a tandem epoxidation/semipinacol rearrangement step to construct a quaternary aza [6.5] spiro ring (rings A and B), an intramolecular aldol reaction to assemble a [3.2.1] bridged ring system, and tandem semireduction/lactonization to form a five-membered lactone D-ring.


 Please wait while we load your content...
Please wait while we load your content...