Kinetic resolution of 2-aryl-2,3-dihydroquinolin-4(1H)-one derivatives by rhodium-catalysed asymmetric transfer hydrogenation†
Abstract
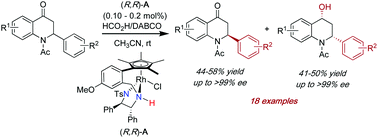
The kinetic resolution of 2-aryl tetrahydro-4-quinolone derivatives was efficiently achieved by rhodium-catalysed asymmetric transfer hydrogenation using HCO2H/DABCO as the hydrogen source. The reaction afforded the enantiomerically enriched 2-aryl-2,3-dihydroquinolin-4(1H)-ones with excellent levels of enantioselectivity (up to >99% ee) as well as the corresponding synthetically useful enantiomerically enriched 2-aryl tetrahydro-4-quinolols in high isolated yields and up to >99% enantioselectivity with high selectivity factors (s up to 1057).


 Please wait while we load your content...
Please wait while we load your content...