Yttrium and lanthanum bis(phosphine-oxide)methanides: structurally diverse, dynamic, and reactive†
Abstract
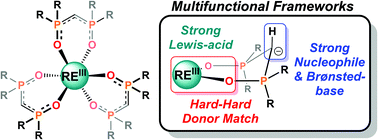
Herein we report the synthesis, characterization, and initial reactivity of homoleptic yttrium and lanthanum complexes of bis(phosphine-oxide)methanides, HRL− (R = Me, Ph; RE(HPhL)3 and RE2(HMeL)6). Our combined experimental and computational studies reveal rich structural diversity dictated by R-group and rare-earth, and the complexes are highly fluxional in solution, even at −80 °C. Computational studies provide quantitative insight into the extensive negative hyperconjugation between the methanide lone-pair and the σ*(P–R) and σ*(P–O). These interactions provide substantial stabilization of coordinated HRL−, yet leave significant charge density at the methanide carbon. The stabilization energies are insenstive to ligand conformation, which contributes to the range of accessible binding modes, structures, and dynamic solution behavior. The largely localized anionic charge leads to strongly nucleophilic and Brønsted-basic reactivity of the methanide. This is demonstrated by the first report of a rare-earth promoted Horner-Wittig reaction and rapid reactivity of Y(HPhL)3 and Y2(HMeL)6 with MeOH/MeOD. The rapid acid–base reactivity of Y(HPhL)3 with MeOH supports a lowering of the effective pKa of a protic Lewis base by >6 orders of magnitude upon binding to Y(HPhL)3, and implies that multifunctional reactivity (Lewis-acid/Brønsted-base) may be possible. Our studies suggest that a rich set of stoichiometric and catalytic reactivity might be realized with these otherwise overlooked fragments.
- This article is part of the themed collection: Rare Earth Chemistry – In memory of Professor Xu Guangxian at his centenary


 Please wait while we load your content...
Please wait while we load your content...