Zeolite-Y encapsulated cobalt(ii) Schiff-base complexes employed for photocatalytic dye-degradation and upcycling CO2†
Abstract
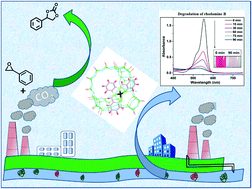
Planar cobalt(II) Schiff-base complexes show modified structural and functional properties after encapsulation inside zeolite-Y. In the free-state, the reactivity of the cobalt(II) Schiff-base complexes is primarily controlled by the electronic effects of the different substituent groups present in the complexes. However, the encapsulation of these complexes within the supercages of zeolite-Y causes the alteration of the electron density around the metal center and subsequently modifies the reactivity. We have intended to study two environment-friendly reactions, i.e., degradation of rhodamine B dye and transformation of CO2 with catalysts containing relatively lower metal content like cobalt(II) salophen complexes encapsulated in zeolite-Y. To comprehend these systems well, XRD analysis, SEM-EDX, FTIR, XPS, TGA, BET, and UV–visible spectroscopy and DFT studies have been performed. XRD and BET studies along with XPS, experimental and theoretical electronic spectroscopic studies clearly revealed successful encapsulation of the guest complex with some alteration of the electronic environment around the metal center. Catalytic studies identified that encapsulated complexes exhibit the better results for dye degradation. Zeolite-Y encapsulated 5-methoxy cobalt(II) sal-1,2-phen (CoL5-Y) could degrade 89.6% of the dye while neat CoL5 could only degrade 30.2%. Comparative studies of structural and catalytic modifications allowed a rational explanation of enhanced reactivity of zeolite encapsulated metal complexes for the degradation of rhodamine B dye compared to their corresponding free-states. The catalysts showing the maximal and minimal activities for dye degradation in the series, i.e., CoL1, CoL1-Y, CoL5, and CoL5-Y, have been tested for coupling of CO2 and styrene oxide. The TON for CO2 uptake using the heterogeneous catalyst CoL5-Y was found to be 2949, while using the homogeneous counterpart, it was only 339.


 Please wait while we load your content...
Please wait while we load your content...