Copper catalysts for photo- and electro-catalytic hydrogen production†
Abstract
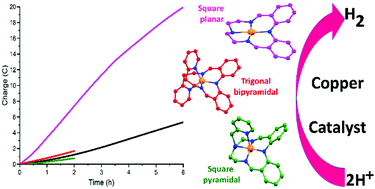
Green production of hydrogen, a carbon-zero future fuel, requires long lived, high activity catalysts made from inexpensive, earth abundant metal ions. Only 15 molecular copper complexes catalyze the H2 evolving reaction (HER). Herein 3 such complexes are prepared and studied as catalysts for both photo- and electro-catalytic HER. Two new N5-donor analogues of the literature N4-donor Schiff base macrocycle HLEt (from [1 + 1] condensation of 2,2′-iminobisbenzaldehyde (dpa) and diethylenetriamine), macrocycle HLEt-MePy (2-bromomethylpyridine alkylation of HLEt) and non-cyclic HLEtPy2 (condensation of dpa and two 2-aminoethylpyridine), were prepared. Then literature [CuII(LEt)]BF4 (1), and new [CuII(LEt-MePy)]BF4 (2) and [CuII(LEtPy2)]BF4 (3), were prepared and structurally characterized, revealing square, square pyramidal and trigonal bipyramidal copper(II) geometries, respectively. Testing under photocatalytic conditions showed that 1–3 have modest turnover numbers (TON = 460–620), but the control, using Cu(BF4)2, had a higher TON (740), and the blank (no copper) also had significant activity (TONequiv = 290). So this is a cautionary tale: whilst 1–3 initially appeared to be promising catalysts for photocatalytic HER, running the control and blank – studies often not reported – shows otherwise. Hence the focus shifted to electrocatalytic HER testing. All three complexes show reversible redox events in MeCN vs. 0.01 M AgNO3/Ag: E1/2 = −1.39 V (1 and 2); −0.89 V (3). Unlike complexes 2 and 3 or the control, 1 is shown to be, or to form, an effective and stable electrocatalyst for HER in MeCN with acetic acid as the proton source (at 100 mV s−1, Ecat/2 = −1.64 V so overpotential necessary for catalysis = 0.23 V, and icat/ip = 34, where icat is peak catalytic current and ip is 1e− peak current for 1 in absence of acid): after 6 hours at −1.6 V, the TON for 1 is 12.5, despite the tiny glassy carbon working electrode used, and it retains good electrocatalytic activity. Results of both ‘rinse and repeat’ (for catalytically active deposit on working electrode) and drop of Hg (for formation of catalytically active nanoparticles) tests are consistent with homogeneous catalysis by 1, but a small copper stripping wave is seen after acetic acid is added, so it is probable that these initial test results are ‘false negatives’, and that there is a heterogenous catalytically active species present; so future studies will probe this point further.
- This article is part of the themed collection: FOCUS: Electrocatalytic Hydrogen Evolution


 Please wait while we load your content...
Please wait while we load your content...