Theoretical insight into the opposite redox activity of iron complexes toward the ring opening polymerization of lactide and epoxide†
Abstract
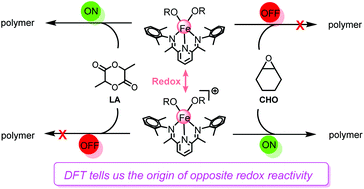
The origin of opposite reactivity in the ring-opening polymerizations of lactide (LA) and cyclohexene oxide (CHO) catalyzed by redox-switchable bis(imino)pyridine iron complexes has been computationally elucidated. It is found that larger geometrical deformation accounts for the lower activity of the oxidized form (Feox) of the iron catalyst toward LA polymerization in comparison with the reduced analogue (Fered) enabling LA insertion with a moderate energy barrier of 27.1 kcal mol−1. In contrast, compared with the Fered species, the higher activity of Feox toward CHO polymerization could be ascribed to the stronger interaction between Feox and CHO moieties, stabilizing the corresponding transition state. This originated from the higher electrophilicity of Feox, which is more sensitive to the binding of the monomer with higher nucleophilicity, such as CHO. Driven by this theoretical understanding, various Feox analogues were computationally modelled by changing the para-substituents of the initial phenoxyls or modifying the backbone of the bis(imino)pyridine ligand to increase the Lewis acidity (electrophilicity) of such complexes. Expectedly, a lower energy barrier is observed in CHO enchainment mediated by the complexes with electron-withdrawing groups. Notably, such energy barriers positively correlate with the LUMO energies of these complexes with various substituents on the initial phenoxyl groups or on the backbone of the bis(imino)pyridine ligand. These results could provide useful information on the development of redox-switchable polymerization systems.


 Please wait while we load your content...
Please wait while we load your content...