Block copolymers comprising degradable poly(2-ethyl-2-oxazoline) analogues via copper-free click chemistry†
Abstract
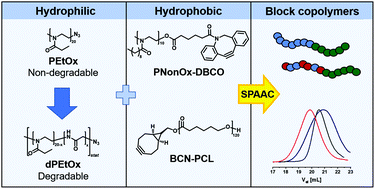
We present the synthesis development of amphiphilic, degradable poly(2-ethyl-2-oxazoline) (PEtOx) analogue block copolymers in a modular fashion utilizing the strain-promoted azide–alkyne cycloaddition (SPAAC). Subsequently to preliminary ω-end group oxidation studies by means of matrix-assisted laser desorption ionization mass spectrometry, a clickable, degradable poly(2-ethyl-2-oxazoline-stat-glycine) was synthesized via a three step post-polymerization synthesis procedure comprising the hydrolysis of azido-terminated PEtOx yielding the linear, azido-terminated poly(ethylene imine), the partial oxidation of the polymer backbone to incorporate randomly distributed glycine moieties and the re-introduction of the N-acyl ethylene imine repeating units. The azido ω-end group enabled the utilization in SPAAC. In a proof of concept approach, the degradable PEtOx analogue and its non-degradable relative were attached to a hydrophobic poly(2-n-nonyl-2-oxazoline) as well as a poly(ε-caprolactone) block via SPAAC. The successful conjugations were confirmed by in depth characterization utilizing NMR spectroscopy, size exclusion chromatography and matrix-assisted laser desorption ionization mass spectrometry. Due to its simplicity and flexibility the presented SPAAC approach offers numerous possibilities for the design of block copolymers comprising degradable poly(2-oxazoline) segments.


 Please wait while we load your content...
Please wait while we load your content...