Alginate-based diblock polymers: preparation, characterization and Ca-induced self-assembly†
Abstract
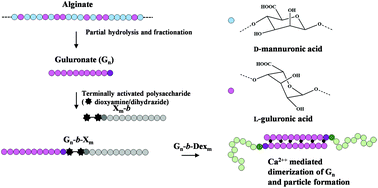
Renewable resources can provide a range of different polysaccharide blocks that can be used to prepare new types of stimuli-responsive polysaccharide-based block copolymers. Alginates are natural polysaccharides widely used as biomaterials. Functional properties depend on the content and distribution of the two 4-linked monomers (β-D-mannuronate (M) and α-L-guluronate (G)). Blocks of L-guluronate (Gn) are responsible for cooperative binding of calcium ions and hydrogel formation. Incorporation of such blocks in block polysaccharide copolymers would represent a new class of engineered, Ca-sensitive biomacromolecules. Dioxyamines and dihydrazides have recently been shown to be well suited for preparation of block polysaccharide structures. Here we first show that when applied to alginate blocks (Gn and Mn) the two types are both very reactive, but the detailed distribution of acyclic (E)- and (Z)-forms and cyclic N-pyranosides, reaction kinetics, conjugate stability, and the rate of Schiff base reduction with α-picoline borane differ considerably, also compared to other polysaccharides. Hence, alginate specific protocols were developed. The linkers introduce a highly flexible joint in otherwise semiflexible Gn-based diblocks. This was demonstrated by SEC-MALS using a symmetrical Gn-b-Gn diblock, which in solution can best be described according to a broken rod model. Ca-Induced self-assembly of Gn-b-dextran diblocks was studied by dynamic light scattering, demonstrating that well defined nanoparticles could be prepared for certain combinations of chain lengths. Taken together, this approach provides a new class of engineered, stimuli-responsive block polysaccharide copolymers solely based on natural resources.


 Please wait while we load your content...
Please wait while we load your content...