Proton conducting ABA triblock copolymers with sulfonated poly(phenylene sulfide sulfone) midblock obtained via copper-free thiol-click chemistry†
Abstract
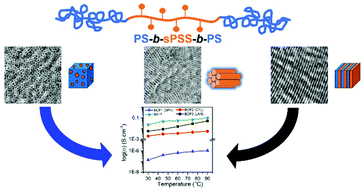
A series of charged ABA triblock copolymers having sulfonated poly(phenylene sulfide sulfone) (sPSS) as B-block and polystyrene (PS) as A-block have been successfully synthesized using copper-free thiol-click chemistry. One-pot sequential radical addition–fragmentation chain transfer (RAFT) polymerization followed by functionalization with a perfluorinated chain extender (decafluorobiphenyl, DFBP) is used to prepare the PS blocks which are later cliked to the charged sPSS mid-block, synthetized using nucleophilic aromatic substitution polymerization. The proposed synthetic approach ensures good control over the composition of the resulting ABA block copolymers allowing synthesis of block copolymers with well-defined ion exchange capacity (IEC) and nanomorphology. The superstrong segregation regime (χN ≫ 100) of these BCPs generates ordered nanostructures, spanning from spherical to lamellar. All the block copolymers are thermally stable up to 300 °C and are robust against swelling and wetting due to the dimensional stabilization of the ionic domains provided by the PS matrix. The relationship between proton conductivity and nanomorphology is investigated by electrochemical impedance spectroscopy (EIS), revealing the significant impact of self-assembly on the transport properties, reaching a maximum ion conductivity of 50 mS cm−1 at 90 °C and 95% RH in the through-plane direction.


 Please wait while we load your content...
Please wait while we load your content...