Low catalyst loading enabled organocatalytic synthesis of chiral bis-heterocyclic frameworks containing pyrazole and isoxazole†
Abstract
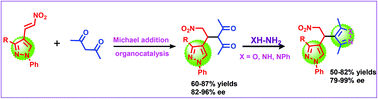
The organocatalytic asymmetric synthesis of enantiopure bis-heterocyclic molecules containing pyrazole and isoxazole under mild reaction conditions has been reported via a low-catalyst loading Michael addition reaction of pyrazolyl nitroalkenes with 1,3-dicarbonyl derivatives. 4-Substituted pyrazole derivatives were obtained in 60–87% yields and with 82–97% ee. These pyrazolyl derivatives are further transformed into chiral bis-heterocyclic derivatives in up to 82% yields and up to 99% ee. The synthesized pyrazole and isoxazole based bis-heterocyclic derivatives are potential bio-active molecules expected to have significant applications. Additionally, the synthesis of these bis-heterocycles can efficiently be carried out in one pot without any loss of enantiopurity, which further adds to its significance.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...