Synthesis of a TNF inhibitor, flurbiprofen and an i-Pr analogue in enantioenriched forms by copper-catalyzed propargylic substitution with Grignard reagents†
Abstract
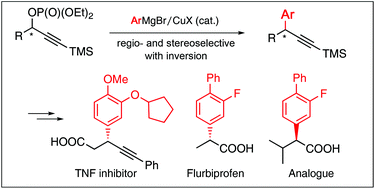
The copper-catalyzed substitution reaction of diethyl phosphate derived from TMSC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH(OH)CH2CH2OTBDPS with 3-c-C5H9-4-MeOC6H3MgBr, followed by several transformations, afforded a tumor necrosis factor inhibitor possessing a Ph-acetylene moiety. The inhibitor was also synthesized from phenylacetylene phosphate PhC
CCH(OH)CH2CH2OTBDPS with 3-c-C5H9-4-MeOC6H3MgBr, followed by several transformations, afforded a tumor necrosis factor inhibitor possessing a Ph-acetylene moiety. The inhibitor was also synthesized from phenylacetylene phosphate PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH(OP(O)(OEt)2)CH2CH2OTBDPS. Furthermore, the substitution of phosphates derived from TMSC
CCH(OP(O)(OEt)2)CH2CH2OTBDPS. Furthermore, the substitution of phosphates derived from TMSC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH(OH)CH3 and TMSC
CCH(OH)CH3 and TMSC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH(OH)-i-Pr with 3-F-4-PhC6H3MgBr gave the corresponding substitution products, which were transformed to flurbiprofen and its i-Pr analogue, respectively. The copper-catalyzed substitutions in these syntheses proceeded in a regio- and stereoselective manner.
CCH(OH)-i-Pr with 3-F-4-PhC6H3MgBr gave the corresponding substitution products, which were transformed to flurbiprofen and its i-Pr analogue, respectively. The copper-catalyzed substitutions in these syntheses proceeded in a regio- and stereoselective manner.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...