Total synthesis of apratoxin A and B using Matteson's homologation approach†
Abstract
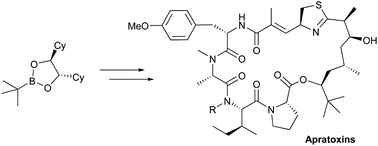
Apratoxin A and B, two members of an interesting class of marine cyclodepsipeptides are synthesized in a straightforward manner via Matteson homologation. Starting from a chiral boronic ester, the polyketide fragment of the apratoxins was obtained via five successive homologation steps in an overall yield of 27% and very good diastereoselectivity. This approach is highly flexible and should allow modification also of this part of the natural products, while previous modifications have been carried out mainly in the peptide fragment.


 Please wait while we load your content...
Please wait while we load your content...