Hypervalent-iodine mediated one-pot synthesis of isoxazolines and isoxazoles bearing a difluoromethyl phosphonate moiety†
Abstract
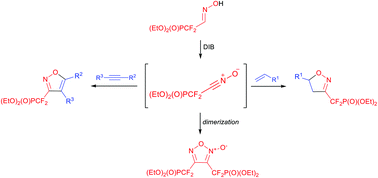
A one-pot, regioselective 1,3-dipolar cycloaddition of in situ generated (diethoxyphosphoryl)difluoromethyl nitrile oxide toward selected alkenes and alkynes is reported. This protocol enables facile access to 3,5-disubstituted isoxazolines and isoxazoles bearing a CF2P(O)(OEt)2 moiety in good to excellent yields, under mild reaction conditions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...