Hydrated metal ion as a general acid in the catalytic mechanism of the 8–17 DNAzyme
Abstract
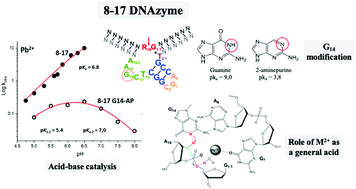
The RNA-cleaving 8–17 DNAzyme, which is a metalloenzyme that depends on divalent metal ions for its function, is the most studied catalytic DNA in terms of its mechanism. By the end of 2017, a report of the crystal structure of the enzyme–substrate complex in the presence of Pb2+ probed some of the previous findings and opened new questions, especially around the participation of the metal ion in the catalytic mechanism and the promiscuity exhibited by the enzyme in terms of the metal cofactor required for catalysis. In this article we explore the role of the divalent metal ion in the mechanism of the 8–17 DNAzyme as a general acid, by measuring the influence of pH over the activity of a slower variant of the enzyme in the presence of Pb2+. We replaced G14, which has been identified as a general base in the mechanism of the enzyme, by the unnatural analog 2-aminopurine, with a lower pKa value of the N1 group. With this approach, we obtained a bell-shaped pH-rate profile with experimental pKa values of 5.4 and 7.0. Comparing these results with previous pH-rate profiles in the presence of Mg2+, our findings suggest the stabilization of the 5′-O leaving group by the hydrated metal ion acting as a general acid, in addition to the activation of the 2′-OH nucleophile by the general base G14.
- This article is part of the themed collections: Editor’s Collection and Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...