Direct functionalization of quinoxalin-2(1H)-one with alkanes: C(sp2)–H/C(sp3)–H cross coupling in transition metal-free mode†
Abstract
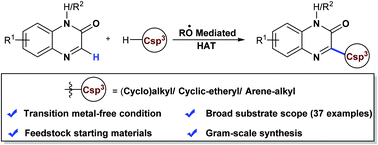
Considering the significance of pharmaceutically important heterocycles, efficient and highly versatile protocols for the functionalization of diverse heterocycles with easily accessible feedstock are crucial. Here, we have reported selective alkylation of quinoxalin-2(1H)-one with a broad class of hydrocarbons having different C(sp3)–H bonds with varying bond strengths using di-tert-butyl peroxide (DTBP) as an alkoxyl radical mediator for hydrogen atom transfer (HAT). This dehydrogenative coupling approach utilizes feedstock chemicals such as cycloalkanes, cyclic ethers and alkyl arenes as coupling partners. This protocol exhibits good functional group compatibility and selectivity regarding both heterocycles and unactivated alkanes. Moreover, this methodology allows functionalization of relatively strong C–H bonds of adamantane and exclusive selectivity towards 3° C(sp3)–H bonds is observed. We also illustrate the applicability of this C(sp2)–H/C(sp3)–H cross-coupling for practical access to bioactive pharmaceuticals.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...